Services
Inquire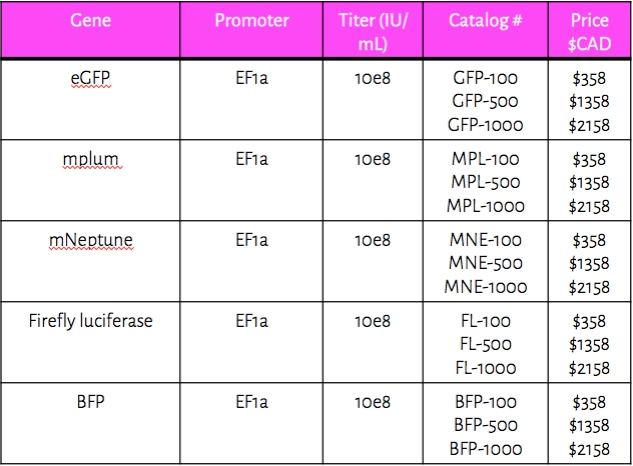
PRE-MADE LENTIVIRUS VECTORS
Tailored Genes provides ready-to-use lentivirus vectors. All vectors contain the EF1alpha promoter, but we can design custom vectors with CMV, hPGK or EFs promoters to meet your individual needs. Please complete our inquiry form if interested in a custom vector.
STABLE CELL LINES
Tailored Genes uses lentiviral vectors to generate stable cell lines. We transfer your gene into a lentiviral vector, then transduce your cell line of choice with the gene. This generates a genetically homogenous, stable cell line where the gene is continuously expressed. Stable cell lines expressing specified genes are essential for use in recombinant protein and antibody production, functional characterization of genes, and drug screening studies.
Custom CRISPR Vectors
We can design and generate custom CRISPR lentivirus vectors to aid you in your gene-editing goals. We can provide gene-specific knockout, repression, and activation lentivirus vectors using CRISPR constructs, allowing you to manipulate gene expression in your desired host cells. Our CRISPR vectors are fully customizable, and we’ll work with you to come up with a design that meets your needs. We understand that CRISPR editing can be used for an infinite variety of research ambitions, and we can collaborate with you to achieve your individual goals.
CONSULTATION & CUSTOM PROJECTS
FULL PROJECT EXECUTION
Let us carry out your full project, from gene amplification/synthesis, vector construction, virus generation, gene expression in target cells, and expression analysis quickly and meticulously. We’ll provide you with regular updates, keeping you informed the entire time. Our priorities are the same as yours: staying on time, within budget, and achieving your individual project goals.
Shipping to the U.S.
An import permit from Centers For Disease Control And Prevention (CDC) is required for shipment of viral vectors to the U.S. An import permit is a document issued by the national government authorizing the importation of infectious biological materials that could potentially cause disease in humans and in order to prevent their introduction and spread into the U.S.
It is the customer’s responsibility to provide the relevant import permit and/or supporting documentation in order to facilitate shipping biological materials into the country. There may also be duties applied by local customs upon receipt. Incorrect or invalid contact information could delay your delivery. Users should consult their official permitting office as these forms are subject to change.
Use the following link to get the import permit from the CDC: https://eipp.cdc.gov/.
A step-by-step guide to walk you through obtaining your permit: https://www.cdc.gov/cpr/ipp/docs/eIPP_agents_training_508.pdf
General
We predominantly manufacture 3rd Generation LV vectors. These 3rd generation vectors are manufactured by transfection of producer cells with 4 expression plasmids, a LV gag-pol expression plasmid, a LV Rev expression plasmid, a VSV-G envelope expression plasmid and an expression plasmid containing the gene of interest.
We can also manufacture 2nd Generation lentivirus vector if requested. The 2nd generation vectors are produced by transfection of producer cells with 3 expression plasmids; a LV gag-pol-rev-tat expression plasmid, a VSV-G envelope expression plasmid, and an expression plasmid containing the gene of interest.
The MOI or the multiplicity of infection represents the ratio of the number of infectious units of virus vector per target cell. Therefore, a MOI of 10 refers to 10 infectious units of vector per target cell.
Vector titers are determined by Q-PCR.
For LV vector titration, genomic DNA isolated from target cells transduced with LV vector, is subjected to Q-PCR to determine the number of integrated LV vector genome using primer and probe specific to the vector sequence. LV vector titers are reported as IU/mL, where IU is infectious units and represents the number of infective viral particles. Since the delivered LV vector is stored at -80°C, the LV vector titer is performed on vector preparations that have been stored at -80°C and thawed.
For AAV vector titration, a small aliquot of AAV vector preparation is subjected to Q-PCR using primers specific to vector promoter. AAV vector titers are reported as GC/mL, where GC is genome copies and generally represents the number of viral particles with a genome. As with LV vector titering, AAV titering is also performed on vector preparations that have been stored at -80°C and thawed.
LV vector kit must be kept frozen and stored at -20°C. Use immediately after thawing and avoid multiple freeze-thaw cycles as it reduces the efficiency of the kit.
